Abstract
Osteoarthritis, the most prevalent joint disease and one affecting many aging adults is strongly associated with various degrees of disability and high health costs. Commonly deemed largely incurable and progressive, it appears muscle fat deposition and its encroachment on muscle tissue may account for multiple adverse health outcomes, especially the osteoarthritic disease process. This mini review examines whether contemporary evidence supports a role for efforts towards preventing excess fat infiltration into vulnerable muscles as one means of reducing osteoarthritic pain and disability. To this end, research on this theme and reported as of June 2025 on this issue was sought. We found that with few exceptions and regardless of joint examined a role for muscle mass infiltration in osteoarthritis disability appears of high clinical significance.
Author Contributions
Academic Editor: Ian James Martins, Principal Research Fellow Edith Cowan University
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2025 Ray Marks
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Osteoarthritis, a prevalent chronic disease affecting one or more freely moving joints and characterized by progressive bone remodelling, articular cartilage degeneration and soft tissue capsular and ligamnetous alterations continues to induce appreciable levels of physical and socioeconomic disability in a high percentage of older adults no matter where they preside 1, 2, 3, 4. Strongly associated with a complex interaction of local and systemic factors, rather than simple ‘wear and tear’ processes 2, the disease is increasingly characterized by multiple muscle alterations including: muscle atrophy, muscle spasm, muscle contractures, muscle fibrosis and pathology. In addition, osteoarthritis appears to be mediated to some degree by the presiding degree of abnormal muscle fat infiltration or fraction.
To this end, this brief specially examines whether, a) the fat muscle ratio is a feature or predictor of severe osteoarthritis, b) possible treatments to counter this, c) the specific importance of its timely identification in older adults at risk for or diagnosed as having osteoarthritis. It was anticipated the literature would reveal a variety of studies highlighting the potential impact of excess muscle fat mass on osteoarthritic pain. It was also believed that if this thesis can be substantiated, a variety of muscle treatment approaches would be found to reduce osteoarthritis pain, including those that can minimize muscle fat gains that are age associated, diet mediated, or reactive and arise in the face of joint pathology and surgery 5, 6.
Methodology
To examine works that might enlighten in the aforementioned regard, the PUBMED data bases largely extending from Jan-June 2025, using the key words: osteoarthritis, pain, sarcopenia and muscle fat mass were sought. Only articles focusing on osteoarthritis and some form of muscle fat attribute and pain as related to osteoarthritis were selected for review. Described in narrative form, are some general ideas and results of diverse current studies directed towards establishing the muscle fat fraction to lean muscle mass ratios in osteoarthritis contexts. The specific topic of interest, sarcopenia was also reviewed. No systematic review was conducted, however, and points made are those that have recently emerged and comport with the author’s 25 years of research showing high degrees of obesity in cases with disabling hip and knee osteoarthritis and the fact that muscle fat mass can be seen in many young adults who are healthy via body impedance measures. The article focuses largely on intramuscular fat mass, as opposed to subcutaneous fat and muscle atrophy or mass that might prove insightful in efforts to foster the well being of many older adults that can be applied at low cost or non invasively.
Accordingly, first I will highlight some general current 2025 evidence reports that allude to recently observed muscle influences or responses in the osteoarthritis disease cycle, rather than all those that have been published to date. The items chosen are those that have a bearing in the author’s view on pain the hallmark of osteoarthritis, namely cartilage shock absorbing tissue degeneration changes and destruction and include the presence or encroachment of fat into the muscles of vulnerable joints. Second, some emerging evidence of a key role for health behaviors as a mediating or moderating pathogenic factor in this respect. Third, clinical implications, possible clinical directives, and future research ideas are presented.
Assumptions
1. Excess muscle fat mass may adversely impact articular cartilage structural features.
2. The absence of adequate nutrition may impact the risk of severe osteoarthritis via its effect on muscle composition, strength, and joint stability.
3. A failure to control body weight may prove injurious to a joint.
4. Lean muscle mass declines and increases in muscle fat ratios may provoke and perpetuate an array of unwanted joint functional outcomes and pain provoking adaptations.
Evidence that fat mass may generate pain in its own right or provoke osteoarthritis progression is potentially supported to date by a host of biomechanical and muscle composition and quality analyses, ultra sonography, imaging studies, basic studies, and biochemical approaches.
Current Specific Findings
While a role for muscle in the osteoarthritis pathogenic cycle is a relatively recent perspective, and increasing numbers of studies confirm its association with symptomatic osteoarthritis, the notion that muscle fat deposition or infiltration may be a potent disease muscle mediator is not widely documented, nor universally applied to a degree commensurate with its possible diverse pathogenic disease manifestations, attributes and associations. In addition, most studies either discuss methods of evaluating muscle composition as this impacts osteoarthritis in a general sense, or detail post surgical muscle fat attributes, both reactive and possibly pathological. However, among the related studies on this issue published as of June 2025 on PUBMED and PubMed Central science repositories, a fair number that focus on obesity and its implications for joint loading, highlight an independent role for muscle adipose tissue composition alterations including muscle fat deposition. Increasing numbers also indicate it may be possible to mitigate this mediating factor in some cases. For example, among these studies a specific role for physical activity 8 and an array of muscle-related structural and functional changes to address sarcopenia a progressive muscle mass declining state as this impacts mobility, and raises pain levels, possibly additively and extensively and sets the stage for inactivity and muscle fat fraction increases 9. Others show a role for deforming contractures, varying degrees of muscle spasm and subnormal vector influences, plus functional changes in muscle biochemistry that have a unique or collective bearing on cartilage viability and that may implicate or lead to muscle fat excesses and infiltration as well as deficits in lean muscle mass 10, 11, 12, 13.
Other data imply that there may be progressively harmful adverse degrees of joint loading that subsequently manifests as fully fledged osteoarthritis, as various degrees of muscle pathology and disordered biochemical expression and/or kinesiophobia or fear of moving due to pain and possible muscle inflammation 14. Collectively, one or more of these suboptimal non physiological pathological disease states appears to have the potential to foster subnormal muscle forces and joint loading or attenuation responses, as well as marring cartilage nutrition processes, and cartilage integrity, and joint stability 15, 16, 17, 18, 19, 20, 21, 22.
Other data reveal observations of prevailing or emergent muscle fat associated metabolic muscle alterations, alterations in muscle quality, thickness, mitochondrial muscle energetics and/or myopathy of the surrounding muscles that may impact on overall physiological reserve and adaptability as well as fostering a perpetual state of undue damage and persistent joint stresses 23, and local signs of muscle weakness. There may also be emergent indications of a gradually diminishing joint range of motion 24, and joint stiffness 25, plus abnormal degree of muscle related joint degrading biomechanics 10, 24, 26. Moreover, it is conceivable that in the absence of effective interventions, as time proceeds muscle tension effects associated with modest intra articular pressure levels, hypoxia and short periods of synovial ischaemia an ongoing cycle of disordered joint destruction and inflammation that causes widespread central pain sensitization or neuropathic states emerges incrementally and persists to destroy the joint as follows: Figure 1
Figure 1.Hypothetical relationships between osteoarthritis pathology, pain and muscle fat deposition factors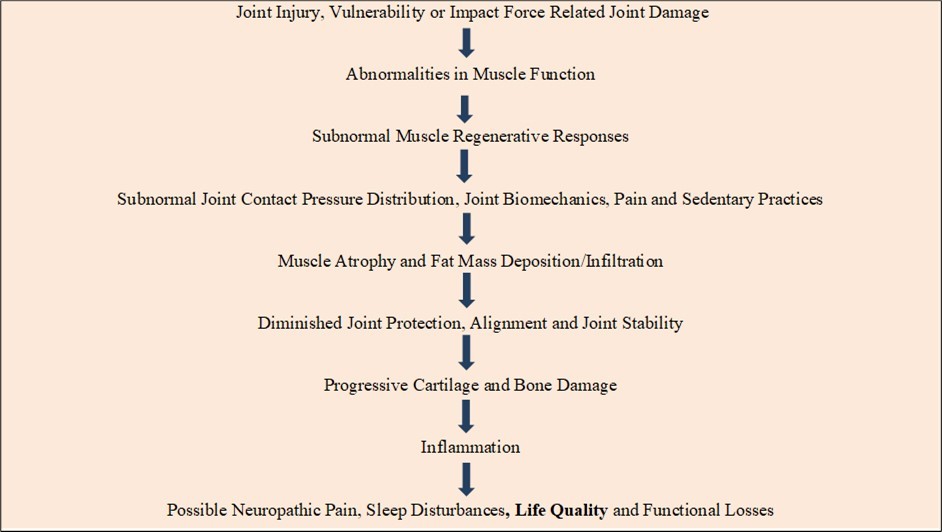
This envisioned series of adverse interactions including heightened muscle strength and contractile abnormalities 26 and others that may induce or be mediated by muscle fat encroachment is indeed increasingly shown to be associated with subnormal joint biodynamical, structural and osteoarthritis joint destruction. For example, in knee osteoarthritis, the most prevalent site of osteoarthritis damage, the disease is often accompanied by a significantly reduced muscle strength capacity and a greater body mass index, wherein a large accumulated fat mass linked with greater systemic inflammation and possible inflammation mediated muscle damage may play a pathogenic role 30. As well, this idea is consistent with known age associated degrees of the gradual onset of subnormal muscle metabolic physiological states, losses of lean muscle mass, increasingly sedentary behaviours, and possibly some neuromotor conditions.
Underlying local factors may also include possible painful muscle reactive adaptations due to persistent abnormal sensory inputs from one or more of the surrounding tissues in the presence of an increased muscle fat mass and parallel decline in muscle mass 15, 16, 17, 18, 19, 20, 30 that may impact the ability to attenuate joint impact loads significantly and effectively as well as muscle quality and architecture 30. Other research suggests persistent muscle spasm resulting from excessive stretching of diseased tissues or abnormally stimulated muscle nociceptors, myokines. or adverse metabolites may also produce a state of ischemic pain in its own right, as well as a state of poor muscle endurance and disabling muscle fatigue that is hard to mitigate 31. Also known as myosteatosis, its presence in muscle exhibits a negative correlation with muscle mass, strength, mobility, and a decrement in muscle quality, while serving as a biomarker for sarcopenia, cachexia, and metabolic syndromes. It also induces proinflammatory changes that clearly contribute to declines in muscle function, compromise mitochondrial function, and increase muscle inflammatory responses 31.
Others show the presence of muscle fat derived inflammation may not only evoke local muscle pain signals, but may elicit more widespread pain, bone attrition, and sensory sympathetic inputs that contribute to the arthritis disease and disability cycle 32. Unsurprisingly, the presence of a decreased quadriceps cross-sectional area and increased intramuscular adipose tissue in knee osteoarthritis has been found predictive of downstream symptom worsening and a need for knee replacement especially in the overweight adult 33, 34.
In this regard, a longitudinal follow-up study of 4.9 years, 472 participants who developed knee osteoarthritis, and 387 cases hip osteoarthritis showed the degree of intramuscular fat in the anterior thigh was significantly associated with incident knee osteoarthritis as well as hip osteoarthritis. Dose-response relationships were also observed. The interaction and subgroup analyses supported the view that this muscle fat effect is a unique pathogenic factor and one that occurs independent of body mass index or gender 34 and may have a bearing on ongoing physical activity challenges as well as emergent alterations in both muscle architecture and degeneration and declines in functional abilities 35, 36, plus exaggerated sympathetic reflex mechanisms and more fatty tissue acquisition than not 37, 38.
It also appears muscle fat fibrosis may have a separate or combined cumulative and progressive effect on osteoarthritis structural pathology indicators 39, 40, 41, and regardless of whether this situation is causative or reactive, unless identified early on, it may be highly challenging to heighten their physical function or relieve longstanding pain or mitigate osteoarthritis, especially if there is a progressive unwillingness on the part of the affected adult to move to counter increasing pain and stiffness, widespread reflex reorganization, body mass increases, alongside muscle mass declines, joint instability, and muscle fiber atrophy. In addition, in the presence of marked instability and/or complete joint stiffening, the abnormal and awkward movements used by the individual to avoid pain and injury may throw strain on other joints, causing further pain and possible reactive diverse muscle associated adverse structural alterations as well as adverse muscle strength implications 42, 43, 44.
Recent data specifically reveal a higher degree of intramuscular fat in both knee extensors and hamstring muscles in middle-aged women with knee osteoarthritis compared with that in a healthy group. It was speculated that weakness of these muscles may to changes in their architectural properties that varies depending on the degree of muscular infiltration. It was concluded the presence of intramuscular fat in the knee muscles is an important determinant of the subjects’ performance and physical function abilities and one worth examining further 44.
Other muscle based determinants that may be related to the degree of muscle fat encroachment and presence involved in osteoarthritis include declines in physical performance and quality of life as well as potential for enhancing the risk for non-communicable diseases, immobility, and disability 45. Increasing data appear to agree upon multiple associated possible harmful degrees of reactive muscle fat deposition in the context of painful osteoarthritis such as subnormal degrees of muscle fibrosis, muscle inflammation, joint instability, and altered cartilage calcium presence 45, 46, 47, 48, 49, 50. Those muscles that may then undergo attrition and/or muscle fat encroachment may also produce slower than desirable reflex reactions that enhance the risk of incurring more extensive joint pathology and pain than those generated in the presence of sufficient fat free lean muscle mass 46, 47, 48, 49, 50.
Recent evidence also shows a possible role for osteoarthritis associated muscle fat infiltration 51, as well gene and protein metabolic alterations 52 that may occur alongside widespread pain, atrophic muscle weaknesses, muscle volume deficits 53 inflammation 54 and muscle architecture alterations including the presence of intramuscular fat deposition or infiltration 55. At the shoulder joint, it appears muscle resident fibroadipogenic cells do serve as a key contributor to rotator cuff tear pathological changes 20.
While mechanical injury is the most likely cause of osteoarthritis in most instances, emerging evidence shows functional ability declines in the presence of excess muscle fat in early knee osteoarthritis symptoms that could be causative or reactive or both 56. An increased muscle fat ratio and its presence can possibly engender multiple pathological states including various degrees of systemic inflammation, physical dysfunction, muscle and muscle metabolic impairments and pain 51, 55, 57, 58. As applied to the vastus medialis muscle of the thigh in knee osteoarthritis, this situation also appears strongly correlated with a decline in the quality of the medial femoral cartilage 58.
As well, those cases displaying atrophic muscle weakness are likely to also display disruptions in muscle genetics, muscle inflammation, and joint instability that may progress to more detrimental muscular alterations such as declines in muscle force capacity and responsiveness 44. Possible central processing neural mechanistic changes that occur over time may further serve to amplify the pain attributable to the local condition even after surgery in the absence of salient timely targeted intervention that embraces efforts to mitigate obesity and possible muscle fatty tissue infiltration and deposition even in the face of joint replacement surgery 76.
Although more research on diverse osteoarthritis samples of higher ages, joints other than the hip and knee joints that predominate is desirable, and more emphasis on muscle pain, inflammation, and atrophy, a role for muscle fat mass correlates that may foster extensive alterations in muscle reactivity and neural signaling at multiple levels including cartilage integrity, is a topic strongly advocated by researchers as a promising one, that cannot be ignored.
In addition, even though the source of fat infiltration and why this may enlarge and become pathogenic is not well clarified, nutrition and physical activity as well as injury appear to play a possible role beyond age and genetics and could serve as basis for intervention approaches that can reduce muscle fat encroachment safely, as well as mitigate-if not reverse- joint destruction, as indicated. As well, multiple diverse intervention modes directed at osteoarthritis pain relief or obesity prevention or both may help as well, especially if they alleviate fears of moving and anxiety.
Currently, as of June 2025, despite the limited body of research that has emerged to date and appears to basically be in its ‘infancy’, it is evident many recent osteoarthritis studies show an enormous potential for studies that explore muscle fat mass deposition and/or its expansion as a potential key pathogenic factor or co-factor requiring attention. Additionally, it appears physical therapies that address this pathogenic disease feature and especially those that can prevent or minimize its impact may greatly help to mitigate this disabling disease. Especially indicated are those strategies that encourage efforts to increase lean muscle mass, strength, power, and careful movements, while reducing pain. Also indicated are: a) weight control; b) possible muscle protein supplements; c) strategies directed towards enhancing muscle reactivity, and efforts towards avoidance of repetitive injurious joint loading activities that induce pain 20, 59, 60, 61, 62, 63, 64, 65. Muscle stress protection in particular seems highly relevant as recent analyses reveal that in the presence of failed regeneration in injured muscle promotes a hostile microenvironment characterized by heightened inflammation, fibrosis, and denervation that may reduce the remaining muscle tissue's quality, and stimulate intramuscular adipose tissue expansion and its known lipotoxic effects on regeneration and contractile function especially in the face of a sedentary lifestyle 66.
At present, even though more solid information on this topic is necessary to acquire, it appears most clinical researchers currently assume muscle problems of some sort underlie and impact osteoarthritis pain and if minimized will prove to have a bearing on osteoarthritis outcomes. In particular, distinctive efforts to study and address muscle fat deposition origins and impacts such as fears of movement 67, muscle strength 68, 72 pain 69, and pain behaviours 74 although rare, are realms of study that will likely prove advantageous as well as insightful. Indeed, increasing attention alone is being paid to muscle fat as a mediator or correlate of osteoarthritis and this realm of cumulative inquiry is adding a new dimension to understanding its origins and progression, and need to obviate or avert its role as a specific site for generating toxic substances with the potential to impede normal muscle function and metabolism and foster potential muscle damage 36, 71 as well as health negating body wide changes due to cross talk between fat and body tissues and pain 74. More specific exploration on how to exploit this emergent set of understandings and their potential for effective osteoarthritis preventive as well as treatment strategies should help to greatly advance the field as cited by most current researchers, even those who conduct joint replacement surgery 76. These observations largely support and reveal a negative role for the presence of fatty tissue in muscles around an osteoarthritis damaged joint as well as how muscle fat mass deposition and enlargement may impact joint biomechanics, muscle parameters such as coordination, flexibility, strength, contraction speed, and endurance adversely in the face of painful osteoarthritis especially in an aging population vulnerable to declines in the lean muscle mass to fat ratio 77, 78.
Discussion
Although osteoarthritis is currently deemed a chronic progressively disabling condition with no known cure, research over the past 10 years or more has indicated that there is strong possibility that an array of muscle related factors as well as obesity can contribute to the osteoarthritis pain and disease cycle.
Conversely, a diverse array of intervention approaches that focus on maximizing muscle structure and function appear advocated to potentially reduce the degree of excess muscle fat mass often noted in this population alongside pain, so as to foster desirable outcomes, regardless of joint site and disease severity.
Excess muscle fat may also impact outcomes of surgery to replace a diseased joint, stressing its importance. Applying what we do know towards primary prevention of injuries, and the adoption of active living rather than sedentary behaviors by many will however likely foster overall joint as well as general health, while helping to reduce enormous public health resource demands from depletion, even in the face of surgical solutions and especially among those in advanced disease stages. Key supportive approaches such as enabling sound dietary practices, electrical muscle stimulation, sensory motor and strength training, and Tai Chi, are likely to prove efficacious as well as safe for many in this regard.
To achieve optimal results, however, a role for cognitions cannot be ignored, given the fact that pain is widespread and fear and depression are readily provoked among those with osteoarthritis and severe pain. Additionally, cases need to be carefully educated as to the considerable care they must take however, to avoid overexertion, muscle fatigue, and repetitive movements, which can heighten muscle pain inputs and accelerate cartilage destruction, as well, as excess sedentary practices. Additional care and careful monitoring to avoid overstretching the joint, and helping those with severe overweight to lose weight is advocated as well. As well, avoiding high frequency loading activities after periods of immobilization found to hasten cartilage destruction is clearly of additional import.
At this point, and despite limitations of this review as well as the state of the research, it appears that while osteoarthritis continues to be described as both inevitable as well as incurable, this immensely painful disabling condition can be mediated or moderated by factors other than age or wear and tear. As such, based on cumulative and emergent evidence, it seems plausible to suggest that the osteoarthritis sufferer’s wellbeing can be effectively mitigated if not reversed if efforts to minimize muscle fat invasion of vulnerable joints is forthcoming, especially if tailored to the overall health status and needs and abilities of the individual and supported by objective measures of muscle composition, force capacity, pain, cognitions, plus weight. In particular, to avert rapid or excess disease progression and disability, and its association with immense social and mobility-related losses, there appears to be an increasing body of research that supports the view that efforts to reduce muscle fat encroachment is a highly salient osteoarthritis disease mitigation strategy. Hence, even if not the key pathogenic cause of osteoarthritis, its prevention must be seen of paramount importance in minimizing osteoarthritic joint pain and dysfunction.
However, since this is by no means a universally accepted idea or practice, more studies that tease out the possible relationship between muscle factors and osteoarthritic pain along with central factors that affect pain and muscle fat encroachment are warranted. Carefully controlled intervention studies with larger samples with similar muscular and disease related characteristics conducted over extensive time periods utilizing a variety of possible interventions could prove insightful as well.
Presently, as per earlier studies, a recent study 66 that strove to characterize lower limb muscle quality in cases with knee osteoarthritis revealed an unanticipated associated of a higher degree of fat infiltration and lower normal-density in the hip muscles rather than the knee extensors (commonly targeted in isolation and thought to predominate the condition). It appears therefore that knee osteoarthritis cases may suffer in part due to associated altered levels of hip abductor and external rotator muscle metabolism alterations, including muscle fat deposition associations, rather than the knee extensors 70. Another that assessed muscle cross-sectional areas, echo intensity, and shear modulus in the knee muscles of 24 knee osteoarthritis cases and 24 controls indicated osteoarthritis muscle and strength losses, poor flexibility and increased passive tension, and possibly reductions in its contractile components and muscle force generating capacity that may accompany knee osteoarthritis 68. Another revealed high levels of intramuscular fat and poorer function than controls 41 with possible significant implications including pathological disease mechanisms, muscle strength, impaired muscle regeneration, and life quality outcomes 71, 72, 73, 74, 75.
In short, although limited, current findings strongly highlight the degree to which muscle fat infiltration and/or enlargement may play a disabling role in the osteoarthritis disease cycle, as well fostering multiple levels of focal and systemic dysfunction, severe pain, and biomechanical and metabolic challenges. Moreover, a failure to appreciate and understand the importance of identifying, tracking, and examining muscle factors such as muscle fat mass in general in the realm of both osteoarthritis research and the design of optimal osteoarthritis rehabilitation plans and their scope and sequence may be shortsighted at best and warrants more careful study and one supported by possible AI diagnostics 31.
Key Conclusions
While recognizing the limitations of this line of inquiry and analysis we tend to conclude:
High degrees of osteoarthritis impairment and suffering will persist among older adults if myths about the conditionand sub optimal intervention approaches remain entrenched.
Efforts to unravel the profound impact of muscle fat mass on joint health as one approach will prove beneficial.
Targeted and tailored intervention studies to prevent fat encroachment and excess weight gain in later life are likely to prove highly efficacious.
Research thatexamines muscles and joints from the perspective of intramuscular fat other than the hip and knee are indicated.
Sarcopenia and obesity prevention are strongly warranted among all aging adults.
A studyof disease free older adults and how muscle fat affects joint loading and muscle regeneration would be revealing and greatly advance the field.
Until more is known, we further conclude that although most of the initiating osteoarthritis determinants that affect many older adults highly adversely are not known and the disease is commonly deemed incurable, it seems possible too that a variety of subnormal bone-muscle interactions and biological signaling alterations that are stimulated by an enlarged muscle fat cell presence can readily impact joint biomechanics as well as joint structure, including muscle and must be considered a key disability determinant, co-factor or osteoarthritis biomarker 63 Moreover, rather than neglecting or overlooking the relevance of possible increases in disordered muscle composition, especially those attributable to injury related fatty infiltration processes, interventions to avert this encroachment or minimize it can help offset a perpetual state of progressive muscle dysfunction, neuropathic pain, cartilage failure and its disabling life quality and functional implications.
We further conclude early detection, and primary prevention here is of high import, rather than late end stage interventions and will avert much suffering, functional disability, and pain in those numerous older adult populations at risk for osteoarthritis disability. In particular, helping vulnerable aging adults to avoid adopting a sedentary lifestyle, educating them about the impact of excess body weight and joint impacts on their wellbeing and need for joint protection, and the importance of regularly participating in exercises that build strength and foster weight control are highly indicated as well.
Final Thoughts
In addressing the growing burden of osteoarthritis, conceptually modelling to unlock and elaborate on the true nature of the condition, a holistic lens that tracks muscle composition and weight status routinely and its joint and life affirming ramifications warrants concerted study and may show how much osteoarthritis suffering can be avoided or undone.
Acknowledgments
none
Conflicts of interest
none
Funding
none
References
- 1.Leszczyński P, Lisiński P, Kwiatkowska B. (2025) Clinical expert statement on osteoarthritis: diagnosis and therapeutic choices. 63(2), 104-115.
- 2.Abramoff B, Caldera F E. (2020) Osteoarthritis: Pathology, Diagnosis, and Treatment Options. MedClin North Am. 104(2), 293-311.
- 3.Ma W, Chen H, Yuan Q. (2025) and national epidemiology of osteoarthritis in working-age individuals: insights from the global burden of disease study 1990-2021.Sci Rep. 15(1), 7907.
- 4.Wang F, Cao Y, Lu H. (2025) Osteoarthritis incidence trends globally, regionally, and nationally, 1990-2019: an age-period-cohort analysis.Musculoskeletal Care. 23-1.
- 5.Halvorson R T, Archibeck E, Khattab K. (2025) Early biomechanical recovery following total hip arthroplasty is associated with preoperative hip muscle fat-fraction.JOrthopRes. 43(6), 1113-1121.
- 6.Tibrewala R, Pedoia V, Lee J. (2021) Automatic hip abductor muscle fat fraction estimation and association with early OA cartilage degeneration biomarkers. J Orthop Res. 39(11), 2376-2387.
- 7.Collins K H, Lenz K L, Pollitt E N. (2021) Adipose tissue is a critical regulator of osteoarthritis.Proc. , NatlAcadSci U S A 118-1.
- 8.He J, Zhang C, Yang L. (2025) Association between sedentary behavior, physical activity, and osteoarthritis: results from NHANES 2007-2020 and Mendelian randomization analysis.Front Public Health. 12, 1454185.
- 9.Whibley D, Shieu M M, Dunietz G L. (2024) Sleep disturbances and progression of mobility disability: longitudinal findings from the Nurses' Health Study.SleepEpidemiol. 4, 100071.
- 10.Yoo J J, Cho N H, Lim S H. (2014) Relationships between body mass index, fat mass, muscle mass, and musculoskeletal pain in community residents.ArthritisRheumatol. 66(12), 3511-3511.
- 11.Kumar D, Karampinos D C, MacLeod T D. (2014) Quadriceps intramuscular fat fraction rather than muscle size is associated with knee osteoarthritis.Osteoarthritis Cartilage. 22(2), 226-34.
- 12.Karapınar M, Ayyıldız V A, Unal M. (2024) Effect of intramuscular fat in the thigh muscles on muscle architecture and physical performance in the middle-aged women with knee osteoarthritis.JOrthopSci. 29(1), 194-199.
- 13.Wang L, Valencak T G, Shan T. (2024) Fat infiltration in skeletal muscle: Influential triggers and regulatory mechanism.iScience. 27(3), 109221.
- 14.Distefano G, Harrison S, Lynch J. (2024) Skeletal muscle composition, power, and mitochondrial energetics in older men and women with knee osteoarthritis.ArthritisRheumatol. 76(12), 1764-1774.
- 15.Visser M, B H Goodpaster, S B Kritchevsky. (2005) Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons.JGerontolABiolSci Med Sci. 60, 324-333.
- 16.Gorski T, Casartelli N C, Fitzgerald G. (2024) Intramuscular fatty infiltration and its correlation with muscle composition and function in hip osteoarthritis.SkeletMuscle. 14-1.
- 17.Siso D, Wee H, Ponnuru P. (2024) The association of rotator cuff muscle morphology and glenoid morphology in primary glenohumeral osteoarthritis.Shoulder Elbow. 17585732241269193.
- 18.Elliott J M, Smith A C, Hoggarth M A. (2020) Muscle fat infiltration following whiplash: a computed tomography and magnetic resonance imaging comparison.PLoSOne. 15-6.
- 19.Gu H, Hong J, Wang Z. (2024) Association of MRI findings with paraspinal muscles fat infiltration at lower lumbar levels in patients with chronic low back pain: a multicenter prospective study.BMCMusculoskeletDisord. 25-1.
- 20.Merriman MA Jr, Chapman J H, Whitfield T. (2025) Fat expansion not fat infiltration of muscle post rotator cuff tendon tears of the shoulder: regenerative engineering implications.RegenEng TranslMed. 11(1), 1-14.
- 21.Verdú E, Homs J, Boadas-Vaello P. (2021) Physiological changes and pathological pain associated with sedentary lifestyle-induced body systems fat accumulation and their modulation by physical exercise.Int J Environ Res Public Health. 18(24), 13333.
- 22.Shinonaga A, Matsumoto H, Tochio K. (2025) Influence of fatty infiltration of muscle on falls and fall-related outcomes in middle-aged and older adults: a systematic review.Cureus. 17-4.
- 23.Negm A, Roberts B, Vette A H. (2025) The effect of sarcopenic obesity on knee biomechanics in individuals with end-stage knee osteoarthritis.Gait Posture. 119-118.
- 24.Ziegelmayer S, Häntze H, Mertens C. (2025) Intermuscular adipose tissue and lean muscle mass assessed with MRI in people with chronic back pain in Germany: a retrospective observational study.Lancet Reg Health Eur. 54, 101323.
- 25.Wu T, Wang X, Cai Z. (2025) Longitudinal associations between baseline sarcopenia and knee osteoarthritis progression and risk of knee replacement.ArthritisRheumatol.
- 26.Gao J, Yesihati M, Cheng H. (2024) Association of sarcopenia and its prognostic value in symptomatic knee osteoarthritis among older people in China: the first longitudinal evidence from CHARLS.BMCGeriatr. 24-1.
- 27.Kwak M K, Baek J Y, Park S J. (2024) Higher circulating resistin levels linked to increased sarcopenia risk in older adults.J Clin EndocrinolMetab. Published online
- 28.Vieira F T, Godziuk K, Barazzoni R. (2025) Hidden malnutrition in obesity and knee osteoarthritis: assessment, overlap with sarcopenic obesity and health outcomes.ClinNutr. 48, 111-120.
- 29.Straatman L, Ivanochko N K, Maciukiewicz J M. (2025) Interaction of obesity and systemic inflammation with knee extensor strength in knee osteoarthritis.ClinBiomech(Bristol).
- 30.George T B, Keret S, Pillai A C. (2025) Fatigue is common in myositis and is associated with disease activity.Semin Arthritis Rheum.
- 31.Garcia-Diez A I, Porta-Vilaro M, Isern-Kebschull J. (2024) Myosteatosis: diagnostic significance and assessment by imaging approaches.Quant Imaging Med Surg. 14(11), 7937-7957.
- 32.Mohajer B, Dolatshahi M, Moradi K. (2022) role of thigh muscle changes in knee osteoarthritis outcomes: osteoarthritis initiative data.Radiology. 305(1), 169-178.
- 33.Weng Q, Jiang T, Yang T. (2025) Association of thigh intramuscular fat infiltration with incident knee and hip osteoarthritis: a longitudinal cohort study.ArthritisRheumatol.
- 34.Joseph G B, Akkaya Z, Sims W M. (2025) MRI-based analysis of thigh intramuscular fat and its associations with age, sex, and BMI using data from the osteoarthritis initiative data.Sci Rep. 15(1), 6188.
- 35.Taniguchi M, Fukumoto Y, Yagi M. (2023) A higher intramuscular fat in vastus medialis is associated with functional disabilities and symptoms in early stage of knee osteoarthritis: a case-control study.Arthritis Res Ther. 25-1.
- 36.Zhang X, Pan X, Deng L, Fu W. (2020) Relationship between knee muscle strength and fat/muscle mass in elderly women with knee osteoarthritis based on dual-energy X-Ray absorptiometry.Int J Environ Res Public Health. 17-2.
- 37.Raynauld J P, Pelletier J P, Roubille C. (2015) Magnetic resonance imaging-assessed vastus medialis muscle fat content and risk for knee osteoarthritis progression: relevance from a clinical trial.Arthritis Care Res (Hoboken). 67(10), 1406-1415.
- 38.Chang J, Liao Z, Lu M. (2018) Systemic and local adipose tissue in knee osteoarthritis.Osteoarthritis Cartilage. 26(7), 864-871.
- 39.Kumar D, Link T M, Jafarzadeh S R. (2021) Association of quadriceps adiposity with an increase in knee cartilage, meniscus, or bone marrow lesions over three years.Arthritis Care Res (Hoboken). 73(8), 1134-1139.
- 40.Aily J B, M de Noronha, Ferrari R J. (2025) Differences in fatty infiltration in thigh muscles and physical function between people with and without knee osteoarthritis and similar body mass index: a cross-sectional study. BMC Musculoskelet Disord. 26-1.
- 41.Baum T, Inhuber S, Dieckmeyer M.association of quadriceps muscle fat with isometric strength measurements in healthy males using chemical shift encoding-based water-fat magnetic resonance imaging. , J Comput Assist Tomogr 40(3), 447-451.
- 42.Chen Z H, Wang Y, Chen W J. (2023) [Relationship between intramuscular fat content in the quadriceps muscle and clinical severity in patients with knee osteoarthritis]. Zhongguo Gu Shang. 36(12), 1147-1152.
- 43.Karapınar M, Ayyıldız V A, Unal M.Effect of intramuscular fat in the thigh muscles on muscle architecture and physical performance in the middle-aged women with knee osteoarthritis. , J Orthop Sci 29(1), 194-199.
- 44.Spanoudaki M, Giaginis C, Mentzelou M. (2023) Sarcopenia and sarcopenic obesity and osteoarthritis: a discussion among muscles, fat, bones, and aging. Life. , (Basel) 13(6), 1242.
- 45.Pan F, Tian J, Scott D. (2022) Muscle function, quality, and relative mass are associated with knee pain trajectory over 10.7 years. Pain. 163(3), 518-525.
- 46.Sepúlveda-Loyola W, Silva-Díaz Y A, Molari M. (2025) Association between the fat mass/fat-free mass ratio and muscle strength, static balance and exercise capacity in older adults: a cross-sectional study. Nutr Hosp. 43(3), 464-469.
- 47.Loureiro A, Constantinou M, Beck B. (2019) A 12-month prospective exploratory study of muscle and fat characteristics in individuals with mild-to-moderate hip osteoarthritis. BMC Musculoskelet Disord. 20-1.
- 48.Taniguchi M, Fukumoto Y, Yagi M. (2023) A higher intramuscular fat in vastus medialis is associated with functional disabilities and symptoms in early stage of knee osteoarthritis: a case-control study. Arthritis Res Ther. 25-1.
- 49.Gorski T, Casartelli N C, Fitzgerald G. (2024) Intramuscular fatty infiltration and its correlation with muscle composition and function in hip osteoarthritis. Skelet Muscle. 14-1.
- 50.Teichtahl A J, Wluka A E, Wang Y. (2015) Vastus medialis fat infiltration - a modifiable determinant of knee cartilage loss. Osteoarthritis Cartilage. 23(12), 2150-2157.
- 51.Seyedhoseinpoor T, Taghipour M, Dadgoo M. (2022) Alteration of lumbar muscle morphology and composition in relation to low back pain: a systematic review and meta-analysis. 22(4), 660-676.
- 52.Snodgrass S J, Stanwell P, Weber K A. (2022) Greater muscle volume and muscle fat infiltrate in the deep cervical spine extensor muscles (multifidus with semispinalis cervicis) in individuals with chronic idiopathic neck pain compared to age and sex-matched asymptomatic controls: a cross-sectional study. BMC Musculoskelet Disord. 23-1.
- 53.Drummer D J, McAdam J S, Seay R. (1985) Perioperative assessment of muscle inflammation susceptibility in patients with end-stage osteoarthritis. , J Appl Physiol 132(4), 984-994.
- 54.Pedroso M G, de Almeida AC, Aily J B. (2019) Fatty infiltration in the thigh muscles in knee osteoarthritis: a systematic review and meta-analysis. Rheumatol Int. 39(4), 627-635.
- 55.Okada S, Taniguchi M, Yagi M. (2025) Degeneration of the cartilage quality is correlated with a higher intramuscular fat infiltration of the vastus medialis in older adults with pre-to-mild knee osteoarthritis. , Eur 183-111930.
- 56.Jiang Z, Wang K, Zhang H. (2025) Correlation between paraspinal muscle fat infiltration and thoracic vertebral degeneration based on phantom-less QCT: a novel insight into thoracic vertebral degeneration. Eur Spine. 34(3), 837-852.
- 57.Teoli A, Martel-Pelletier J, Abram F. (2022) Vastus medialis intramuscular fat is associated with reduced quadriceps strength, but not knee osteoarthritis severity. Clin Biomech (Bristol). 96, 105669.
- 58.Huynh T, Kim J T, Dunlap G. (2020) In vivo testing of an injectable matrix gel for the treatment of shoulder cuff muscle fatty degeneration. J Shoulder Elbow Surg. 29-12.
- 59.Prokopidis K, Varanoske A N, Veronese N. (2025) Effects of exercise with or without a hypocaloric diet on intermuscular and intramuscular fat: a systematic review. Aging Clin Exp Res. 37-1.
- 60.Al Saedi A, Duque G, Stupka N. (2021) Targeting intramuscular adipose tissue expansion to preserve contractile function in volumetric muscle loss: a potentially novel therapy? Curr Opin Pharmacol. 58, 21-26.
- 61.Wesselink E, E de Raaij, Pevenage P. (2019) Fear-avoidance beliefs are associated with a high fat content in the erector spinae: a 1.5 tesla magnetic resonance imaging study. Chiropr Man Therap. 27-14.
- 62.Teoli A, Martel-Pelletier J, Abram F. (2022) Vastus medialis intramuscular fat is associated with reduced quadriceps strength, but not knee osteoarthritis severity. Clin Biomech (Bristol). 96, 105669.
- 63.Luo J, Xiang Q, Lin T. (2025) Associations between total and regional fat-to-muscle mass ratio and osteoarthritis incidence: a prospective cohort study. Osteoarthritis Cartilage.
- 64.Holm P M, Blankholm A D, Nielsen J L. (2024) Effects of neuromuscular control and strengthening exercises on MRI-measured thigh tissue composition and muscle properties in people with knee osteoarthritis - an exploratory secondary analysis from a randomized controlled trial. Semin Arthritis Rheum. 65, 152390.
- 65.Kitamura G, Nankaku M, Kawano T. (2025) Characteristics of skeletal muscles in lower limb from the perspective of muscle quality in patients with knee osteoarthritis. , J Orthop Res
- 66.Huang Y, Wang L, Luo B. (2022) Associations of lumber disc degeneration with paraspinal muscles myosteatosis in discogenic low back pain. Front Endocrinol (Lausanne). 13, 891088.
- 67.Liu T, Xie H, Yan S. (2025) Thigh muscle features in female patients with severe knee osteoarthritis: a cross-sectional study. BMC Musculoskelet Disord. 26-1.
- 68.González-Gutiérrez J, López-Gómez J J, Primo-Martín D. (2025) Relationship between body composition parameters and quality of life in patients with obesity and osteoarthritis. Nutrition. 135-112765.
- 69.Snodgrass S J, Stanwell P, Weber K A. (2022) Greater muscle volume and muscle fat infiltrate in the deep cervical spine extensor muscles (multifidus with semispinalis cervicis) in individuals with chronic idiopathic neck pain compared to age and sex-matched asymptomatic controls: a cross-sectional study. BMC Musculoskelet Disord. 23-1.
- 70.Zhu Y, Hu Y, Pan Y. (2024) Fatty infiltration in the musculoskeletal system: pathological mechanisms and clinical implications. Front Endocrinol (Lausanne). 15, 1406046.
- 71.Chen L, Zhou H, Gong Y. (2024) How do muscle function and quality affect the progression of KOA? A narrative review. Orthop Surg. 16(4), 802-810.
- 72.Inhuber S, Sollmann N, Schlaeger S. (2019) Associations of thigh muscle fat infiltration with isometric strength measurements based on chemical shift encoding-based water-fat magnetic resonance imaging. Eur Radiol Exp. 3-1.
- 73.Fitzgerald G, Turiel G, Gorski T. (2023) MME+fibro-adipogenic progenitors are the dominant adipogenic population during fatty infiltration in human skeletal muscle. Commun Biol. 6-1.
- 74.Verdú E, Homs J, Boadas-Vaello P.Physiological changes and pathological pain associated with sedentary lifestyle-induced body systems fat accumulation and their modulation by physical exercise. , Int J Environ Res Public Health 18(24), 13333.
- 75.Theret M, FMV Rossi, Contreras O. (2021) Evolving roles of muscle-resident fibro-adipogenic progenitors in health, regeneration, neuromuscular disorders, and aging. Front Physiol. 12, 673404.
- 76.Genc A S, Agar A, Güzel N. (2023) Evaluation of psoas muscle atrophy and the degree of fat infiltration after unilateral hip arthroplasty. Cureus. 15-7.